Details of the Drug
General Information of Drug (ID: DML2NIB)
| Drug Name |
Aminophylline
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aminocardol; Aminodur; Aminofilina; Aminofillina; Aminophyllin; Ammophyllin; Cardiofilina; Cardiomin; Cardophylin; Cardophyllin; Carena; Cariomin; DOBO; Diaphilline; Diaphyllin; Diaphylline; Diophllin; Diophyllin; Diuxanthine; Ethophylline; Eufilina; Euphyllin; Euphylline; Euphyllinum; Eurphyllin; Euufilin; Euufillin; Genophyllin; Grifomin; Inophylline; Lasodex; Linampheta; Lixaminol; Metaphyllin; Metaphylline; Minaphil; Miofilin; Neophyiline; Norofilina; Peterphyllin; Phylcardin; Phyllindon; Phyllocontin; Phyllotemp; Somophyllin; Stenovasan; Syntophyllin; Theodrox; Theolamine; Theolone; Theomin; Theophyldine; Thephyldine; Truphylline; Vasofilina; A 1755; BY 108; Minomal(TN); Phyllocontin (TN); TH/100; Truphylline (TN)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Bronchodilator Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
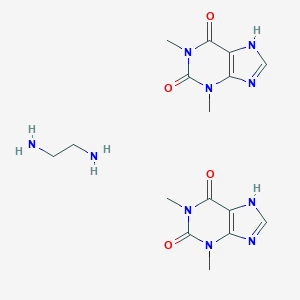 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 420.43 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Aminophylline (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
